2022
GMI listed on the Taiwan FDA food ingredient

GMI (Ganoderma microsporum immunomodulatory protein)is the culmination of 30 years of biotech research in Taiwan.
Here, we witness the metamorphosis of GMI, from the legendary medicinal roots of ganoderma, a traditional Chinese medicine, to the scientifically proven effectiveness of GMI today.


2022
GMI listed on the Taiwan FDA food ingredient
2021
GMI completed review for GRAS by US FDA
2021
GMI found to calm cytokine storms during severe infections
2020
GMI listed on the International Nomenclature of Cosmetic Ingredients (INCI), #35581
2020
GMI listed on the US FDA New Dietary Ingredient (NDI), #1133
2017
GMI found to repair neurons in the brain
2017
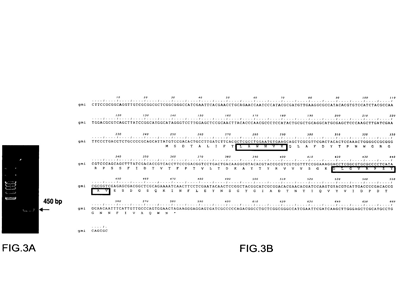
Analytical method of GMI passes TAF-ISO17025 accreditation
2014
Taiwan Ministry of Economic Affairs granted GMI as a potential new drug candidates
2013
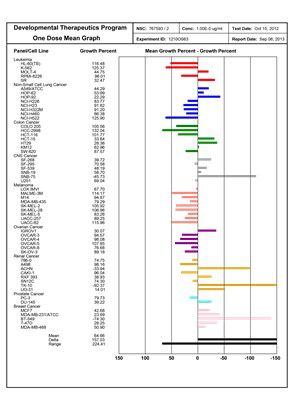
US NIH reports GMI can effectively treat 60 types of common cancer cells
2010
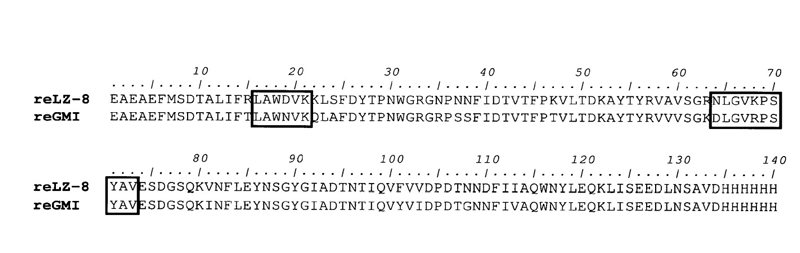
3D structure of GMI registered in Protein Data Bank
2010
GMI found to treat lung cancer cells
2009
GMI registered in NIH database
2005
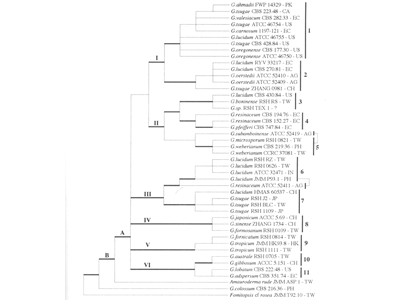
GMI discovered in Ganoderma microsporum using bioinformatics and genetic engineering
1989
New type of ganoderma officially named “Ganoderma microsporum”
1982
A new type of ganoderma mushroom discovered in Taiwan
There are many lab-claimed anti-cancer ingrediants, but few that make it all the way to your doctor’s prescription, because it is very hard to achieve the trifecta of “safety”, “effectiveness”and “mass production”.
Yet today, GMI, an immunomodulatory protein originating from Ganoderma, has achieved just that! A US National Institute of Health (NIH) study has shown the suppressive effects of GMI on 9 types of cancer; Research teams from the National Taiwan University, National Yang-Ming University, Chung Shan Medical University, Taipei Medical University and others have also shown GMI, even at very small doses, to be effective against many different types of cancer, including drug resistant cancer cells and even cancer stem cells. GMI has even been shown to boost the effectiveness and reduce the negative side effects of chemotherapy, targeted therapy and immunotherapy.
Most importantly, all these anti-cancer effects can be safely realized on people.

8F.-1, No. 12, Ln. 270, Sec. 3, Beishen Rd., Shenkeng Dist., New Taipei City 222 , Taiwan (R.O.C.)
+886-2-2662-0148